How LSU Chemists Are Learning from Nature to Rethink Nitrogen Fixation
May 15, 2025
At Louisiana State University, chemists are uncovering how nature efficiently converts nitrogen gas into ammonia. They aim to unravel the inner workings of nitrogenase, a natural enzyme, paving the way for cleaner, more energy-efficient alternatives to traditional fertilizer production.
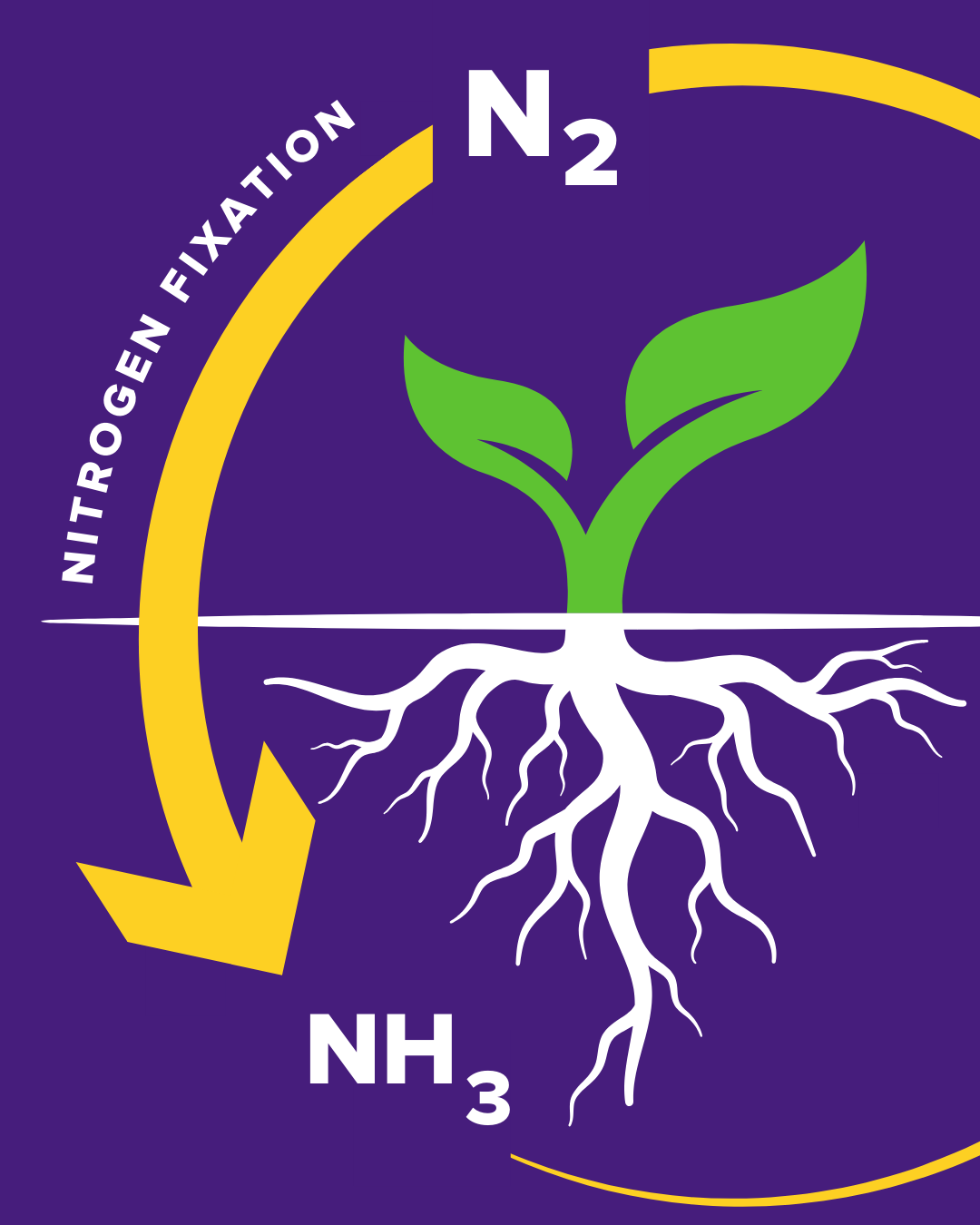
The nitrogen cycle starts with nitrogen fixation, a process driven by the enzyme nitrogenase, which catalyzes the conversion of nitrogen gas into ammonia, enabling plants to grow, animals to eat, and ecosystems to thrive.
Nitrogen is one of the essential elements on which life depends. However, while nitrogen is abundant in the atmosphere as nitrogen gas (N₂), this form is largely inert and unusable by most living organisms.
To be biologically useful, nitrogen must be converted into ammonia (NH₃), a form that living systems can incorporate into vital biomolecules like DNA, RNA, and proteins.
Nature solves this challenge with nitrogen fixation, where the enzyme nitrogenase in certain bacteria and archaea catalyzes the conversion of atmospheric nitrogen into ammonia. Without nitrogen fixation, plants wouldn’t be able to grow, and neither would the animals and humans that depend on them.
To meet global food demands, industrial ammonia production relies on the century-old Haber-Bosch process to produce synthetic fertilizers essential for agriculture. While effective, the process requires high temperatures and pressures and consumes much energy.
Understanding how nature’s nitrogenase drives nitrogen fixation could lead to the development of more sustainable and energy-efficient methods for ammonia synthesis.
LSU Chemists Lead the Way
At the LSU Department of Chemistry, Professor Emeritus Brian Hales and his collaborator, Distinguished Instructor Kresimir Rupnik, focus on understanding how nitrogenase works, specifically, how its intricate metal clusters are assembled and operate.
Nitrogenase is a complex enzyme that contains two metal centers essential to its function: the P-cluster, responsible for electron transfer, and the FeMo cofactor, responsible for the chemical transformation of nitrogen to ammonia. Hales and Rupnik employ advanced spectroscopic tools to investigate these complex metal clusters.
With Magnetic Circular Dichroism (MCD), they identify the type of metal clusters present and reveal their magnetic and electronic properties. Meanwhile, Electron Paramagnetic Resonance (EPR) helps characterize the clusters’ structures and behaviors. The complementary techniques allow them to examine how nature builds and utilizes these metal clusters during nitrogen fixation.

FeMo Cofactor of Nitrogenase
Over the years, Hales’ research produced several significant findings that advanced the field. In 1986, Hales and colleagues reported the discovery of a vanadium-based nitrogenase, containing a FeV cofactor. The variant expanded the known diversity of nitrogenase enzymes and opened new pathways for studying how different metals can influence biological catalysis.¹
In 2011, Hales and Rupnik published a study in the Journal of the American Chemical Society that offered critical insights into the electronic behavior of iron-sulfur clusters, which are central to nitrogenase and many redox-active proteins across biology.²
Their 2014 work further demonstrated that complex metal clusters like the P-cluster could be assembled outside of enzymatic systems. The study provided evidence for the involvement of all-ferrous [Fe₄S₄]⁰ intermediates and suggested possible synthetic pathways for replicating these biologically important structures.³
Hales and Rupnik’s foundational discoveries continue to shape our understanding of one of nature’s most efficient and intricate chemical transformations. By revealing how nitrogenase assembles and operates, their research provides a blueprint for developing greener, energy-efficient industrial processes inspired by nature.
To learn more about the research, visit the faculty webpages of Professor Emeritus Brian Hales and Dr. Kresimir Rupnik.
- Hales, B. J., Case, E. E., Morningstar, J. E., Dzeda, M. F., & Mauterer, L. A. (1986). Isolation of a New Vanadium-Containing Nitrogenase from Azotobacter vinelandii. Biochemistry, 25, 7251–7255.
- Rupnik, K., Lee, C. C., Hu, Y., Ribbe, M. W., & Hales, B. J. (2011). [4Fe4S]²⁺ Clusters Exhibit Ground-State Paramagnetism. Journal of the American Chemical Society, 133, 6871–6873.
- Rupnik, K., Lee, C. C., Wiig, J. A., Hu, Y., Ribbe, M. M., & Hales, B. J. (2014). Nonenzymatic Synthesis of the P-Cluster in the Nitrogenase MoFe Protein: Evidence of the Involvement of All-Ferrous [Fe₄S₄]⁰ Intermediates. Biochemistry, 53, 1108–1116.